Over-the-counter multinutrient drink could have beneficial impact on Alzheimer’s, new study reveals
pharmafile | October 31, 2017 | News story | Manufacturing and Production, Research and Development | Alzheimer's, Fortasyn Connect, Souvenaid, dementia
LipiDiDiet, a European project investigating “the impact of nutritional lipids on neuronal and cognitive performance in ageing, Alzheimer’s disease and vascular dementia”, has unveiled its findings on the efficacy of Fortasyn Connect – also known as Souvenaid – an over-the-counter multinutrient drink which claims to be able to delay the development of Alzheimer’s disease.
The trial encompassed 311 prodromal Alzheimer’s patients across 11 sites in Germany, Sweden, Finland and the Netherlands, randomised to receive either Fortasyn Connect of an iso-caloric control drink for 24 months.
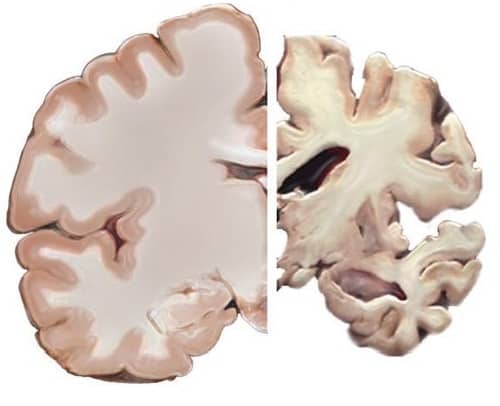
The results found that the trial failed to meet its primary endpoint of improvement in neuropsychological test battery (NTB) score over the two years. However, it was found that cognitive decline was 45% lower than anticipated according to the Clinical Dementia Rating-Sum of Box (CDR-SB) – a measure which determines how the disease affects patients in their daily lives – leading the report to note that this rendered the primary endpoint “inadequately powered”. Additionally, 26% less brain atrophy was recorded in the hippocampus and 16% less in ventricular volume.
“Today’s results are extremely valuable as they bring us closer to understanding the impact of nutritional interventions on prodromal Alzheimer’s, which we are now better at diagnosing but unable to treat due to a lack of approved pharmaceutical options,” said study leader Professor Hilkka Soininen, a neurologist at Eastern Finland University. “The LipiDiDiet study illustrates that this nutritional intervention can help to conserve brain tissue and also memory and patients’ ability to perform everyday tasks – possibly the most troubling aspects of the disease.”
Co-ordinator Prof Tobias Hartmann, of Saarland University in Germany, added: “While this nutritional intervention is not a cure for Alzheimer’s, it effectively shows the earlier in the disease process we intervene, the greater the advantage for the patient.
“Importantly, reduced atrophy in the patient’s brain shows the benefit extends beyond symptomatic effects, something never before achieved.”
This study marks the third clinical trial to show the benefits if the nutritional intervention on memory performance, with the first two studies investigating its effects on patients with mild Alzheimer’s dementia.
Matt Fellows
Related Content

Vesper Bio reports positive topline results for dementia candidate
Vesper Bio, a clinical-stage biotech developing novel oral therapies for neurodegenerative and neuropsychiatric disorders, has …
Lilly’s drug for early Alzheimer’s shows promising results
Eli Lilly (Lilly) has announced positive new data from the long-term extension of its phase …

Mental health medicine use in England reaches record high, NHSBSA report reveals
According to new data published by the NHS Business Services Authority (NHSBSA), mental health prescriptions …






