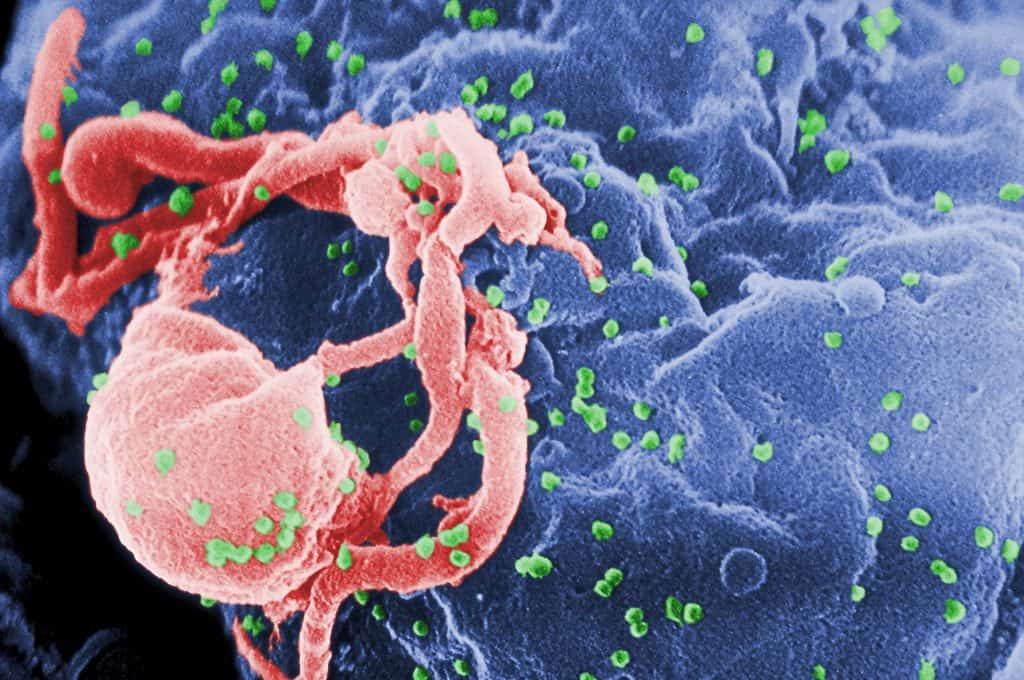
Trump administration block NIH researchers from using foetal tissue, disrupting research to cure HIV
pharmafile | December 10, 2018 | News story | Sales and Marketing | HIV, Trump, US government, feotal tissue, mice, research
The Trump Administration has halted the acquisition of new human foetal tissue for use in experiments conducted by NIH researchers, thus disrupting research into a cure for HIV.
Donald Trump’s administration imposed the suspension without an announcement last September, it was revealed, as interventions into federally funded research using human foetal tissue were expanded. The move has come in response to pressure from anti-abortion campaigners who oppose the use of the tissue which is collected from elective abortions.
The ruling will have profound implications for many researchers, particularly for those who use ‘humanised mice’ – mice who have been altered using foetal tissue thus giving them immune systems that behave in a similar manner to humans. Those looking into HIV will be particularly affected.
One HIV researcher Warner Greene commented: “We were all poised to go and then the bombshell was dropped. The decision completely knocked our collaboration off the rails. We were devastated”
He made the announcement to his lab via email in noting: “[HHS] has directed me to discontinue procuring fetal tissue from ABR, the only source for us. I think that they are the only provider of fetal tissue for scientists in the nation who don’t have direct access to aborted fetal tissue. This effectively stops all of our research to discover a cure for HIV.”
The decision reflects badly on the Trump administration who have been accused of holding ‘anti-science’ views, particularly in relation to climate change.
Louis Goss
Related Content

European Commission approves HIV prevention injection
The European Commission (EC) has granted marketing authorisation for Gilead Science’s Yeytuo (lenacapavir), the first …

HIV vaccine candidate successfully optimised for industrial production
Sumagen Canada Inc, a biotech company based in both South Korea and Canada has partnered …

Drug discovery and development partnership announced between Apollo Therapeutics and Oxford University
Portfolio therapeutics company Apollo Therapeutics has announced earlier this week that it will provide capital …






