UK launches COVID-19 vaccination drive with first dosings of Pfizer/BioNTech’s candidate
pharmafile | December 8, 2020 | News story | Manufacturing and Production, Sales and Marketing | COVID-19, UK, Vaccine
The first doses of Pfizer and BioNTech’s approved COVID-19 vaccine have been administered in the UK in a historic occasion dubbed “V-Day”, less than a year after the SARS-CoV-2 virus emerged in the world.
The first wave of 800,000 doses of the vaccine has hit around 50 selected hospitals and healthcare hubs across the country, following the Medicines & Healthcare Products Regulatory Agency (MHRA)’s decision to approve the candidate for emergency use last week.
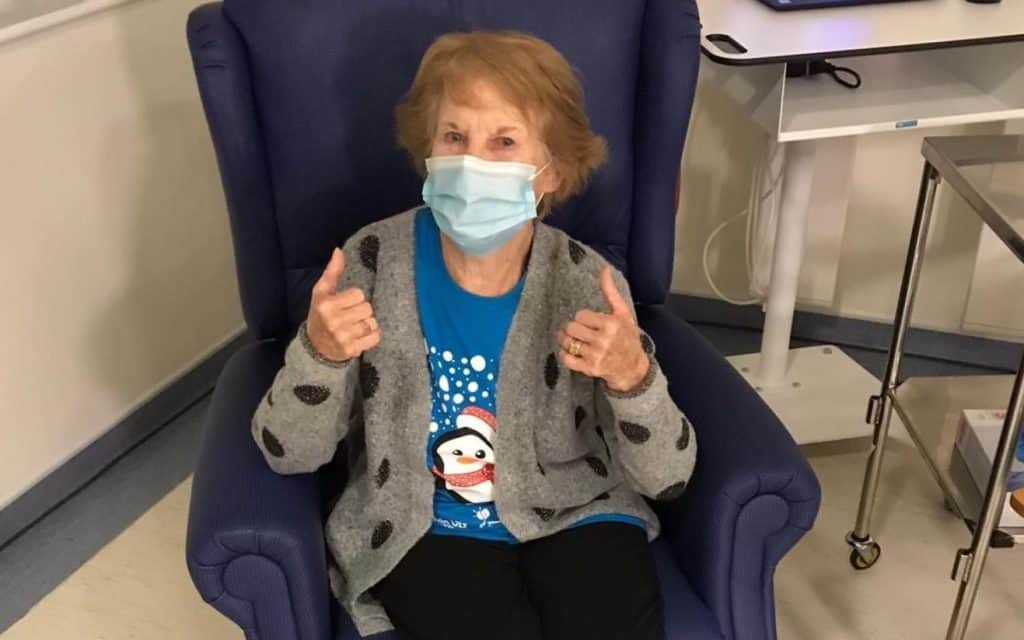
Elderly patients over the age of 80 and frontline healthcare workers have been prioritised to receive the vaccine first. Margaret Keenan (pictured), a 90-year-old grandmother of four from Northern Ireland, was the first patient in the world to be dosed with the vaccine at 6:30am UK time, and more continue to receive their dose as the day goes on.
“I feel so privileged to be the first person vaccinated against COVID-19,” Keenan said after receiving the jab. “It’s the best early birthday present I could wish for because it means I can finally look forward to spending time with my family and friends in the new year after being on my own for most of the year.”
The UK Government aims to roll out the administration of four million doses of the vaccine by the end of the year. In total, it has secured 40 million doses of Pfizer and BioNTech’s two-dose candidate – enough to inoculate 20 million of the UK’s population of almost 67 million. So far, the country has recorded around 1.74 million active cases, and has seen over 51,000 deaths – the highest death toll in Europe.
Matt Fellows
Related Content

Gilead’s Veklury recommended by NICE for COVID-19 treatment
Gilead Sciences has announced that the National Institute of Health and Care Excellence (NICE) has …

NICE expands access to Paxlovid for 1.4 million people at risk of severe COVID-19
The National Institute for Health and Care Excellence (NICE) has announced that it has expanded …

GSK shares new data for RSV vaccine Arexvy
GSK announced positive results from its phase 3 trial which assessed the immune response and …








