FDA ‘yes’ to inhaled insulin
pharmafile | June 30, 2014 | News story | Manufacturing and Production, Sales and Marketing | Afrezza, FDA, Mannkind, diabetes, insulin human
The FDA has approved the first ‘ultra-rapid-acting’ mealtime insulin therapy available in the US, to improve glycemic control in both type 1 and type 2 diabetes sufferers.
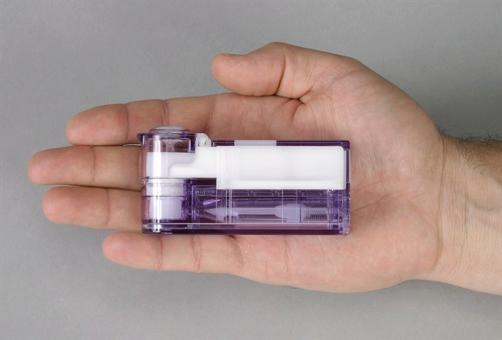
The US regulator approved Afrezza (insulin human) Inhalation Powder, a rapid-acting inhaled insulin which is taken at the beginning of each meal, or within 20 minutes of starting to eat.
The brand, which consists of Afrezza inhalation powder delivered using an inhaler, was given the thumbs-up in April by one of the FDA’s advisory committees.
The powder dissolves immediately upon inhalation into the lungs and delivers insulin into the bloodstream in around 12-15 minutes – compared to 45-90 minutes for injected rapid acting insulin analogs and 90-150 minutes for injected regular human insulin, according to the firm.
“Afrezza is a new treatment option for patients with diabetes requiring mealtime insulin,” says Jean-Marc Guettier, director of the Division of Metabolism and Endocrinology Products at the FDA’s Center for Drug Evaluation and Research.
“Today’s approval broadens the options available for delivering mealtime insulin in the overall management of patients with diabetes who require it to control blood sugar levels,” he adds.
And such options are needed: an estimated 25.8 million people in the US – more than 8% of the population – have diabetes, a disease which has serious health implications including heart disease, blindness and kidney damage.
The product is not a substitute for long-acting insulin and must be used in combination with long-acting insulin in patients with type 1 diabetes.
MannKind will be celebrating because it has been far from plain sailing for the Connecticut-based firm to reach this stage: the initial license application for Afrezza was turned down by the FDA in 2011.
The regulator says additional clinical trials would need to be conducted before it would look again at approving the drug – these include a treatment group using the previously studied MedTone inhaler, in order to obtain a head-to-head comparison of the pulmonary safety data for the two devices.
MannKind will also have to carry out various post-marketing studies as a condition of FDA approval.
Adam Hill
Related Content

Roche’s Alecensa approved by FDA as lung cancer treatment
Roche has announced that the US Food and Drug Administration (FDA) has approved Alecensa (alectinib) …

GSK’s meningococcal vaccine candidate accepted for FDA review
GSK has announced that the US Food and Drug Administration (FDA) has accepted for review …

FDA grants ODD to Candel Therapeutics’ pancreatic cancer treatment
Candel Therapeutics has announced that the US Food and Drug Administration (FDA) has granted Orphan …







