J&J acquires Neuravi to bolster neuro business
pharmafile | April 11, 2017 | News story | Manufacturing and Production, Sales and Marketing | Johnson & Johnson, Neuravi
Johnson & Johnson’s neurosurgery and neurovascular unit, Codman Neuro, has made the move to acquire Galway-based company, Neuravi. The exact pricing of the deal has not been released but is understood to be the largest for a venture-backed medtech in Europe since 2009, as reported by the Irish Times. This places the deal in hundreds of millions and represents a huge success for the company and its investors.
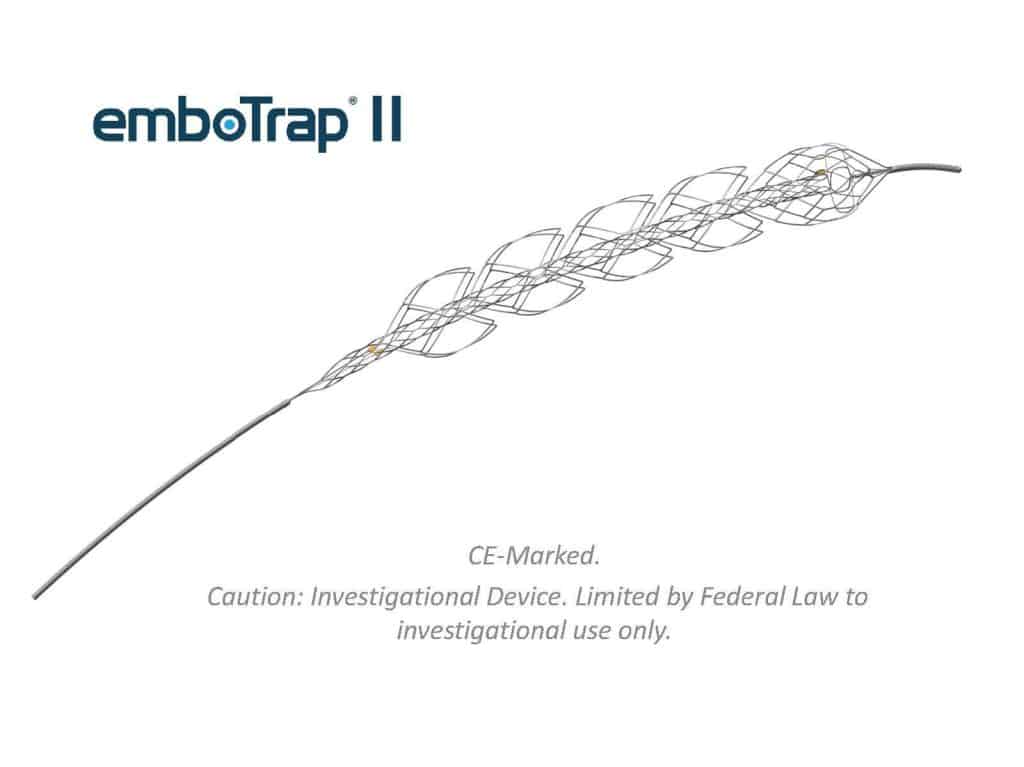
Neuravi’s key product is named emboTrap II and works to remove blood clotting in patients suffering from acute ischemic strokes, which accounts for 87% of all strokes. One million people suffer from this type of stroke every year in Europe and the device has already played a role in 3,000 patients’ treatment.
The device itself, in a crude description of its action, acts as a kind of feather duster for clots. It is inserted through the clot and then the ‘petals’ (see image above) of the device unfurl, from its retractable casing allowing the device to retrieve and remove a blood clot, whilst also picking up any fragments of clot disturbed by the device.
“Rapid restoration of flow is of utmost importance when treating stroke patients,” said Shlomi Nachman, Company Group Chairman of Johnson & Johnson Medical Devices Cardiovascular & Specialty Solutions. “The EmboTrap platform was designed to address this critical need and we are excited to combine Neuravi’s expertise in clot research with Codman Neuro’s global resources to accelerate innovation in acute ischemic stroke treatment.”
The company will continue to operate out of, and expand from, its current Galway base. The product is currently going through US trials and will receive an FDA decision by the end of the year. In Europe, the product currently holds 30% of the market against its rivals in Medtronic and Stryker.
Ben Hargreaves
Related Content

Johnson & Johnson to acquire Shockwave Medical
Johnson & Johnson (J&J) and Shockwave Medical have announced that they have entered into a …

FDA approves J&J’s Opsynvi for PAH treatment
Johnson & Johnson (J&J) has announced that the US Food and Drug Administration (FDA) has …

Johnson & Johnson acquires Ambrx Biopharma for approximately $2bn
Johnson & Johnson (J&J) has announced that it has successfully completed its acquisition of Ambrx …








